Optical Quartz in Maine
Prepared for the Geological Department
At The University of Maine Augusta
Optical Quartz in Maine
Prepared for the Geological Department
At The University of Maine Augusta
Independent study
By
Eric W, Bradstreet
Student, University of Maine
Augusta
24 June 2000
Eric Bradstreet
P.O. Box 1332 ~ Waldoboro ~ Me. 04572
Home Phone 207-832-2159 ~ Email [email protected]
Bob Doyle
258 Civic Center, UMA
Augusta, ME. 04330
Dear Professor Doyle,
I am very intrigued by the challenge we
discussed on June 13, 2000 on the subject of the localized optical quartz in
Maine. Since getting the go ahead for this study I have gathered as much
research that has been done in this area, in hopes to get a feel for the
definition of optical quartz. The research has not revealed a clear definition
in terms of chemical components and structure. The study ahead will provide a
clearer definition of optical quartz.
My research will include a collaboration of
theory around this topic. The forces need to create such an environment that
produces this result (optical quartz). Also it will include the chemical
composition need for optical quartz.
The study will provide an understanding of the
Sebago Batholith and its intricate piece in the development of this localized
occurrence. The aspects of geology that will be discussed are magma, solution
composition, Granite Plutons, and Mineralology.
Some historical information will be discussed,
but the depth of the study will take a scientific approach!
For a more in-depth look at the organization of
this research I have provided an outline index.
Sincerely,
Eric Bradstreet
Student,
University of Maine Augusta
EB
Acknowledgments
I
would like to recognize the assistance I have received in this study. I want to
thank Bob Doyle, science professor at University of Maine at Augusta for the
opportunity of doing this study. His experience in bedrock geology of Maine has
helped this study immensely. Appreciation also goes to the Geological
Department of Maine. Their years of work and compiled information have been a
great asset. I also acknowledge credit too Woodrow Thompson, of the Maine
Geological Department for assisting in the direction of information and is wide
knowledge of minerals in Maine.
Outline
Optical Quartz and its Localized
Occurrence
I).
Preface
II). History
of optical quartz in Maine
A.
Era of
mining quartz
B.
Uses of
optical quartz
C.
Sebago
Batholith
D. How the melting origin affected and interacted with the country rock
III). Geological setting
A. Brunswick Topsham
B. Alburn
C. Alburn
- Sangerville formation
IV). Pegmatite
A. Magma
B. Arteries
C. Veins
D. Solidification
E. Pegmatite
Core Dynamics
V). Overview of quartz and its properties
A. Outline
of quartz
B. Applying
Paulings rule to optical quartz
C. How
did these conditions reach the optimum environment for optical quartz?
D. More
discussion on solidification
E. Past
studies that have been done with quartz
F. Today’s
studies
VI). Summary and Conclusion
A. Tying
all the data together
B. Reiterating
the key issues that brought forth scientific evidence
C. Conclusion
of the study
Preface
This study
focused toward optical quartz and is formatted for the general science
students. The study will explore the optical quartz occurrences in western
Maine.
The work that
has been cited in this study is referenced by Author’s last name at the end of
this document.
Note: The
author's background in geology extends over a 5-year period. Theories set forth
in this study are theories of the author.
The study of
the earth's rocks is known as the science of geology. This science covers many
fields of expertise, earth science, mineralogy, chemistry, physics, and
technology (mapping, compiling data).
This study will
observe the environment best conducive for optical quartz [SiO2]
One of the most abundant mineral in the earth and has many different spectrums [see,
pg.13, Quartz properties]. In
order to understand why quartz produces many spectrums, it is necessary to
explore its environment. This will encompass bedrock geology, thermal geology,
and magma.
One particular
type of quartz used in industry is optical quartz. The study will define the
term optical quartz. Various studies of others work in this field will be used
to compile a synopsis. However the new work in this study will come from the
focus on the eastern flank of the Sebago Pluton, located in South Western
Maine. It is the hope of the author that upon reading this study one will go away
with a clear understanding of optical quartz and its occurrence in Maine.
History of optical quartz
Era of mining quartz:  During the beginning of the industrial
age, quartz became part of the fabric of the electrical industry. In an interview with Geoffrey LaChance the owner of the LaChance quarry located in
Brunswick Maine, He remembers the mining era very well. Geoffrey tells about
when he was just 7 years old, in 1919, the American Glue Company began to
purchase quartz from areas in Maine. The use of this quartz was to make
sandpaper. He remembers it like yesterday, the hard labor of extracting quartz
was time consuming and labor intensive. The price per pound then was unknown to
Geoffrey. It was, F. Thomas LaChance, Geoffrey father who had opened the
quarry. No other quarry produced the large amounts of fine quality quartz like
the LaChance quarry did [Photo on right]. The quarry closed in 1922 and
reopened in 1926 when the General Electric Co. (GE) began to purchase quartz
for its optical value. “The price was $10.00 a ton”(Lachance, 2000. pers. comm.).
Prompting other area prospects to open quarries that were previously mined for
gems or feldspar and began mining them for quartz. Some of the other quarries
that produced and sold optical grade quartz to GE were, “The Pulsifer quarry
located in Auburn Maine, which was mine for gemstones in 1901” (US Bureau of
Mines, 1975. p. 72). “Norway Maine was another location that GE had bought
optical quartz from; the quarry opening was on cobble hill” (LaChance, 2000. pers.
comm.). In the report Maine Pegmatite Mines and Prospects, Maine Geological
Survey of 1957 reported GE also operated its own quarries in Albany and
Buckfeild Maine, both quarries produced optical grade quartz. Upon investigation
of other quarries with in the boundaries of the eastern flank of the Sebago
Batholith; much of the quartz is adulterated with the exception of the LaChance
quarry. It will be to our benefit to investigate why the quartz became
adulteress in these areas and not so adulteress in other areas of purchasing.
The issue of adulteress quartz will be discussed in-depth later in this study.
During the beginning of the industrial
age, quartz became part of the fabric of the electrical industry. In an interview with Geoffrey LaChance the owner of the LaChance quarry located in
Brunswick Maine, He remembers the mining era very well. Geoffrey tells about
when he was just 7 years old, in 1919, the American Glue Company began to
purchase quartz from areas in Maine. The use of this quartz was to make
sandpaper. He remembers it like yesterday, the hard labor of extracting quartz
was time consuming and labor intensive. The price per pound then was unknown to
Geoffrey. It was, F. Thomas LaChance, Geoffrey father who had opened the
quarry. No other quarry produced the large amounts of fine quality quartz like
the LaChance quarry did [Photo on right]. The quarry closed in 1922 and
reopened in 1926 when the General Electric Co. (GE) began to purchase quartz
for its optical value. “The price was $10.00 a ton”(Lachance, 2000. pers. comm.).
Prompting other area prospects to open quarries that were previously mined for
gems or feldspar and began mining them for quartz. Some of the other quarries
that produced and sold optical grade quartz to GE were, “The Pulsifer quarry
located in Auburn Maine, which was mine for gemstones in 1901” (US Bureau of
Mines, 1975. p. 72). “Norway Maine was another location that GE had bought
optical quartz from; the quarry opening was on cobble hill” (LaChance, 2000. pers.
comm.). In the report Maine Pegmatite Mines and Prospects, Maine Geological
Survey of 1957 reported GE also operated its own quarries in Albany and
Buckfeild Maine, both quarries produced optical grade quartz. Upon investigation
of other quarries with in the boundaries of the eastern flank of the Sebago
Batholith; much of the quartz is adulterated with the exception of the LaChance
quarry. It will be to our benefit to investigate why the quartz became
adulteress in these areas and not so adulteress in other areas of purchasing.
The issue of adulteress quartz will be discussed in-depth later in this study.
Uses of optical quartz: In 1921 Professor Walter G. Cady
discovered that quartz could be used to control radio oscillator circuit (US,
Bureau of Mines, 1975 p. 881). In1926
GE began to buy optical grade quartz. The quartz was sorted at the quarries by
looking for clarity within each piece. 100,000 tons were extracted from the
LaChance quarry, but only 8-10 tons were shipped to Lynn Massachusetts
(LaChance, 2000. pers. comm.). There the quartz was sorted again and only the
finest of the quartz was sent to GE’s science department in Schenectady New
York. The remainder was used for electrical purposes. Elihu Thomson, a scientist, head of the science department in
Schenectady was attempting to use the quartz to build a refractive lens. His
attempts failed, because the process thus far, was to solidify the quartz and
pour into a mold, but upon cooling the quartz would crack. What Elihu developed
was Pyrex. The composition of Pyrex is water and quartz fused together. “Fused Silica is synthetic molten
quarzglass. The very good optical transmission characteristics in the UV
(ultraviolet) range besides a high transmission up to the infrared are only two
of the unique properties of this material” (Prazisions, 2000). The properties
used here describe the term optical quartz by the transmission of light
patterns; the UV range. The atomic properties of optical quartz will become
clear throughout this study.
Geology of the Sebago Batholith: The Sebago Pluton is a two-mica granite that intruded the metasedimentary rocks of the Central Maine Terrane around 292 Ma (Behn, Eusden, Notte, 2000). “The ambient temperature at emplacement of the Sebago Batholith is constrained to be approximately 350 degrees C and 500 degrees C” (Tucker and Marvinney 1989. vol.3 p. 27). Tucker and Marvinney also state that the average discordance between muscovite and biotite ages is 5 ma implying that, on average the region cooled at a rate of 15 degrees C/MY between approximately 245 Ma and 230 Ma. Gauging the time that cooling took place determining when each mineral solidified out is part of the process of developing a theory of what conditions were present to form the optical quartz surrounding the Sebago Batholith.
The Sebago Batholith is surrounded by many small
granite plutons. Perhaps these smaller plutons are from the origin melt of the
Sebago Batholith.. This research includes the surrounding pegmatite, the
pegmatite’s are known to be granite pegmatite in this region of Maine. This
would suggest that the batholith be not constrained to the Sebago area.
Although the textures and some composition are different and irregular
surrounding the batholith, they are more alike then different; exspecially in
regards to mineral composition. A study of these rock structures has shown that
the two mica type of the Sebago Batholith is consistent with the surrounding
pegmatite. At the center of the intrusion the texture of the pluton is on a
micro-scale to a granular scale. But as we move outward from the core solution
the texture changes. The granite pegmatite of western Maine have a larger
crystal structure then the pluton localized in the Sebago area. The mineral
composition changes and differs from the core solution of the pluton in these
surrounding pegmatite’s.
There have been many theories why the structure
differs and many studies have been done to prove that they differ; not
excluding the work that has been done to show their likeness. “One man who has
studied these granites and pegmatites has theorized that the inconstancies
reflect that the core solution and rare earth elements in the pematites are
caused by the re-melting of the metamorphic rock” (Laverdeire, 2000.pers. comm.). This suggests that the
batholith extend outward into the country rock. Figure (A ) shows the
positioning of solutions developing within country rock and its
fracture-filling replacement. “These rocks collectively represent an intrusive
history spanning time from the early Devonian to the Cretaceous.… within an
estimated area of 2000 km 2” (Hussey, 1981.File.
no. 81-29).
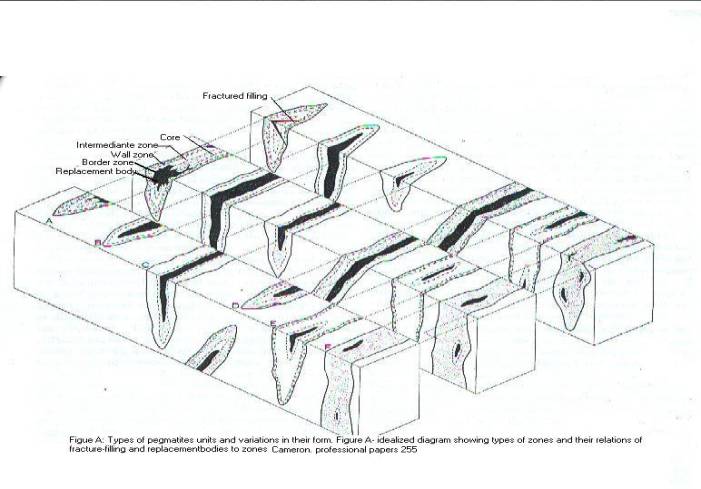
Figure A, by: Cameron, 1942-1945, Pegmatite
Investigation:
New England Geological Survey,
Professional Paper 255 P. 27
Other smaller granite bodies are present in the
surrounding country rock. The origin is from the core of the Sebago Batholith
according to this study (see figure B). The evidence that shows this is that
the mineral composition is much the same in other plutons. If we exclude the
fact that adulteress rock (adulteress
rock , solution carried through metamorphic rock contaminating the origin)
being carried into these surrounding plutons then perhaps what is left is the
same core solution of the Sebago Batholith. “Geophysical evidence suggest that
with the exception of the outer core the earth normally consist of solid
material. Thus any magma must originate by the melting of preexisting solid
rock” (Cox, 1979 p.5). Gravitative separation, flowage differentiation, and
systems involving liquid and vapor are models in which one will arrive at these
determinations.
Needless to say the rock we see today has
undergone many changes while spanning time. The primary composition has not
changed since emplacement of these bodies. Some alteration has occurred due to
movement of tectonic plates, fractures, cracks, and minor re-melting due to
pressure.
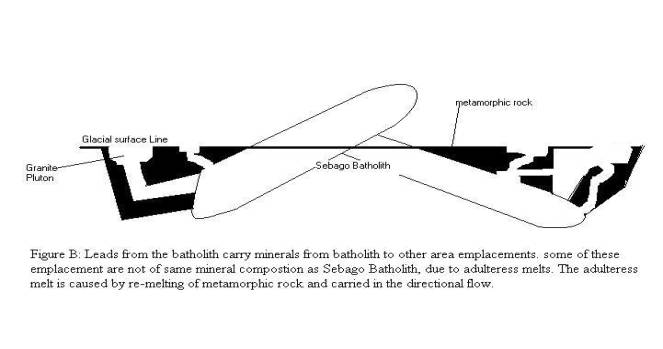
Diagram By: Bradstreet
Geological Setting
Brunswick: (see figure C) The Brunswick area is dated Cambrian --Ordovician, and
is situated within the Cushing formation (Geologic Map. Maine, 1985).
In
the Yarmouth-Brunswick-Garndiner area the cushing formation can be divided into
the Richmond corner, Mount Ararat, and Nehumkeag Pond member…. The Mount Ararat
Member consist of 2-10 cm alternating bands of light gray
quartz-plagioclase-biotite granofels or gneiss, and dark gray biotite-rich
amphibolite with extensive intervals of the light gray gneiss (Hussey 1981. p. 4)….Brookins and Hussey interpret Rb/Sr whole rock ages of the cushing formation to
reflect the age of volcanic activity, and not of a metamorphic event (Hussey, 1981.
p. 5)
The
Lanchance quarry is located within these series of rocks. At the quarry most of
the mineral structures are uniform- absent of adulteress materials-not to imply
that other minerals are not present. Fractionation is minimal; the body
of the pluton protected movement within the core of the pegmatite. Little
re-melting took place after emplacement, keeping the minerals intact today as
they were upon emplacement. Unlike the other structures visited.
Topsham: (see figure C) The fisher quarry lies within Mount Ararat Member, dated
Cambrian --Ordovician, and is part of the Cushing formation also (Geologic Map.
Maine, 1985). “In contrast to the majority of Topsham pegmatites, the Fisher
quarry is undoubtedly the most fractionated pegmatite in the area…the area lies
just west of the Norumbega fault” (Francis, Wise, 1992. p.85) where great
pressure had built up. The Fisher Quarry " is one of the few places in the
area to contain replacement units and pocket" (Francis, Wise, 1992. p.85).
The facts presented here will build a theory of why Optical quartz is more
prevalent in one area then another.
Alburn: The Lewiston and
Auburn area is part of the Sangerville formation. Age: Late Ordovician(?) to
late Silurian (Hussy, 1981. p.8). This study will focus on where quartz was
purchased Mount Apatite. The environment at Mount Apatite is consistent with
granite

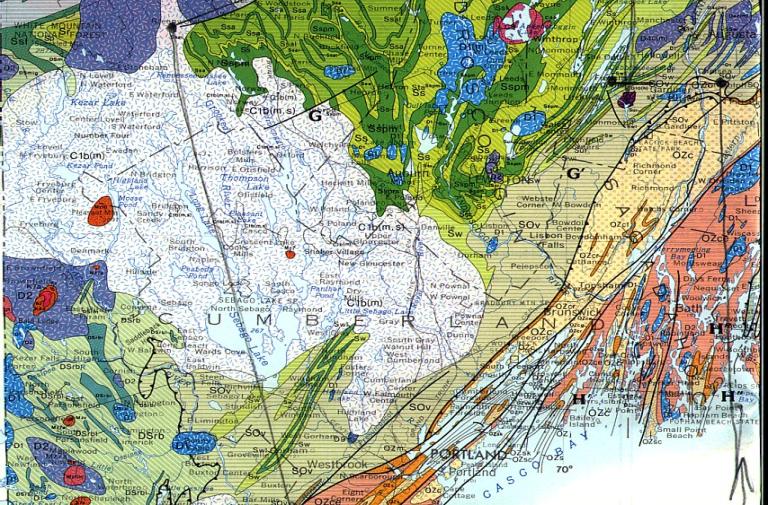

Figure C: Geologic Bedrock Map, Maine, 1985,
Department of Conservation. Edited by: Philip H. Osberg University of Orono,
Arthur M. Hussey Bowdoin College, Gary M, Boone, Syracuse University
pegmatite then fine-grained granite. It is comprised
of mostly quartz, felspar, and mica and is surrounded by metamorphosed rock.
Mount Apatite is noted for its fine green tourmaline and purple apatite that
was discovered in 1901 in the famous Pulsifer Quarry.
Pegmatite
Magma, Arteries, Veins and Solidification: The pegmatite in the location listed
above seems to range from simple pegmatites to very complex. The origin is
still undetermined, but many theories have been adopted. Using the Bowen’s
reaction series, as presented in the publication Understanding Earth By: Siever. Applying this
theory would suggest that magma produced from the Sebago Batholith gave rise to
this pegmatite. The theory further explains the dynamics of solution separation
and at what temperature minerals solidify first and last within magma injected
into the country rock. Magmatic differentiation pertains to the rise and fall
of the parent magma and what stages crystallization (temperature) takes place.
For our purposes we will explore this theory in-depth, because there is much to
be understood how optical quartz solidifies. What conditions where present to
enable the pegmatite’s to produce optical quartz?
The Brunswick,
Thopsom, Alburn country rock area has been filled with magma that has been
differentiated by the collection of other minerals, hence the solution needed
to create the pegmatite’s. These other minerals were collected by the
solidified magma passing through the country rock. While the liquid was
traveling outward from the parent magma chamber [Sebago Batholith] carrying
minerals away and mixing with country rock creating a new magma solution
composition. Meanwhile this process was
depleting the parent magma solution [see
figure B]. “As the process continued, both melt and crystals gradually
became richer in sodium and poorer in calcium” (Siever. 1997. p.87). The
calcium rich magma was transported to the pegmatite chambers. With the parent
magma solidifying, the magma injections slowed into the country rock as it
cooled. Eventually flowage of magma from the parent magma was blocked off [see figure D] and mineral separation
began in the pegmatite chambers that surrounded the Sebago Batholith. However
with the exception of the LaChance Quarry most area pegmatities experienced
later intrusions. These later intrusions introduced great pressure which
partially re-melted metamorphic rock as the injection filled cracks and fissures.
The Thopsom area has a number of granite quarries, which would indicate large
intrusions. [discussed later in this study, mineral seperation]
The evidence
brought forth from the field studies of these areas has revealed that within
the complex pegmatite systems, rare earth elements are present. The presents of
rare mineral crystals such as albite, tourmaline, apatite, and garnet, in these
areas, are consistent with mineral separation that was interrupted by later
intrusions. Another relevant aspect of this theory is that cooling must have
took place over thousands of years. As the magma cooled, taking a plastic form,
pressure and solution moving into the parent magma then reheated the chamber
and its arteries. The magma squeezing outward into the pegmatite chambers
introduced new elements that produced impurities that adulterated the mineral
structures already in place. The pressure of this later intrusion contributed
to the fractionation and some re-melting took place. Areas in the Brunswick 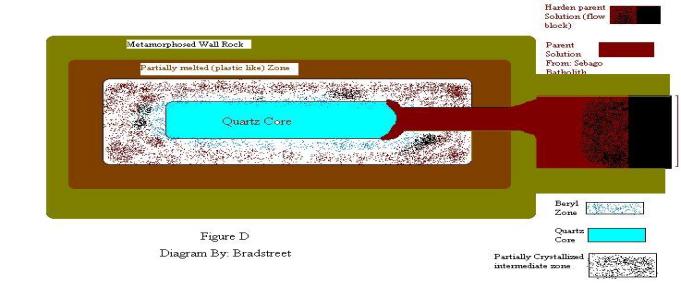
area did not show evidence of these
later intrusions. Leading to the notion that pressure and adulteress mineral
was not a key factor in the development of the optical quartz. This is not to
say that rare earth elements are not present here, because they are. The theory
being developed here is, the pegmatite chamber located in Brunswick [LaChance
quarry] had one injection of magma that cooled over a very long time.
Pegmatite
Core Dynamics: First
lets explain further the dynamics of core solution within pegmatite. “Not
unlike the parent chamber, there is a core, where the solution cools
last”(Doyle, 2000). Normally these solutions are made up of quartz. The diagram
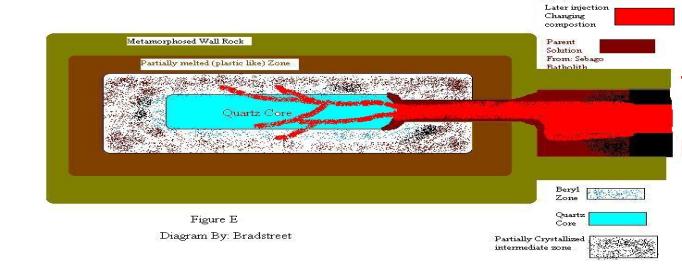
in figure D explains this dynamic process, the LaChance quarry. Figure E shows
the contrast to figure D, Figure E Mount Apatite [Auburn Maine.] and Fisher
Quarry [Thopsom Maine.]. Although the dynamics may differ from location to
location, the models shown in figure D and E are to be used as a general
conception of core dynamics.
In
diagrams E, Mount Apatite site, shows the creation of this pegmatite. The
construction happens by successions of injection into the surrounding country
rock. These later injections were once again forced throughout the country
rock. The pressure, new solution and heat from this force began the process of
alteration to the already harden pegmatite zone. The results are that the
existing minerals in place have been adulterated by these injections. The
forces mention here have many variables in how the minerals are altered. The
first being that more oxygen is supplied to the pegmatite, this can accelerate
cooling. Another element of accelerated cooling is that the pegmatite had
already begun to harden, hence the new injection was met by lower temperature
rock. Rapid cooling produces impurities such as oxygen bubbles. The abundance
of the oxygen bubbles found at Mount Apatite is relatively high as is true for
Thopsom area pegmatite [Fisher Quarry]. Optical quartz exists in these areas on
a much lower scale then at the LaChance quarry. The reason for this is because
the later injections did not reach the center of the quartz core within the
pegmatite. And this is where most of the optical quartz is found. The reason
for relatively small amounts of optical quartz came from areas is because part
of the quartz core did not become adulterated.
In contrast to these events, the
LaChance quarry shows no evidence of this adulteress material. In fact it shows
evidence that the pegmatite in this area remained stable after the primary
injection. A large area outcrops of granite, surrounds and protects the area
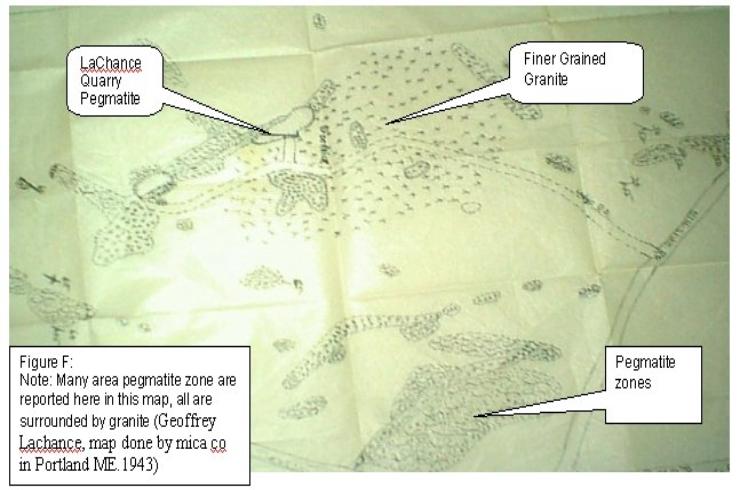
pegmatite (see figure F, p.14). This
surrounding granite may in fact be the parent solution that produced this
particular pegmatite, or it’s, a main artery from the Sebago Batholith that
became block. A later injection found new routes that produced the chamber of
magma. In any event one could surmise that this artery was block early in the
development of the Sebago Batholith (see
figure D). If this is so then the depletion of minerals that produced the
pegmatite may have came from this blocked magma chamber.
Note: Thopsom, and Alburn pegmatities
are surrounded by metamorphosed rock. The Thopsom area has a number of granite
quarries that produce large enough quartz veins that were prospected for it
glassy quartz and gem Beryl.
Having
eliminated pressure and adulteress material as an agent in production of
optical quartz at the LaChance quarry, leaves us to peruse formation of optical
quartz on a molecular and composition level. To get a better understanding of
quartz and its properties, an outline is provided below.
Overview of Quartz and its Properties : Quartz SiO2
Amethyst Galleries, Inc (provides the outline below, 2000).
THE MINERAL QUARTZ
·
Chemistry:
SiO2 , Silicon dioxide
·
Class:
Silicates
·
Subclass:
Tectosilicates
·
Group:
Quartz
·
Uses:
silica for glass, electrical components, optical lenses, abrasives,
gemstones, ornamental
stone, building stone, etc.
·
Additional variety specimens include:
·
Amethyst
is the purple gemstone variety.
·
Citrine
is a yellow to orange gemstone variety that is rare in nature but is
often
created by heating Amethyst.
·
Milky Quartz
is the cloudy white variety.
·
Rock crystal
is the clear variety that is also used as a gemstone.
·
Rose quartz
is a pink to reddish pink variety.
·
Smoky quartz
is the brown to gray variety.
PHYSICAL CHARACTERISTICS:
·
Color
is as variable as the spectrum, but clear quartz is by far the most common
color
followed by white or cloudy (milky quartz). Purple (Amethyst), pink
(Rose Quartz), gray or brown to
black (Smoky Quartz) are also common.
Cryptocrystalline varieties can
be multicolored.
·
Luster
is glassy to vitreous as crystals, while cryptocrystalline forms are usually
waxy
to dull but can be vitreous.
·
Transparency
crystals are transparent to translucent, cryptocrystalline forms can
be
transparent, translucent or opaque.
·
Crystal
System is trigonal; 32
·
Crystal
Habits are again widely variable but the most common
habit is
hexagonal
prisms terminated with a six sided pyramid (actually two
rhombohedrons). Three of the six
sides of the pyramid may dominate
causing the pyramid to be or
look three sided. Left and right-handed crystals
are possible and identifiable only if minor trigonal pyramidal faces
are
present. Druse forms (crystal
lined rock with just the pyramids showing) are
also common. Massive forms can
be just about any type but common forms
include botryoidal, globular,
stalactitic, crusts of agate such as lining the
interior of a geode.
·
Cleavage
is not present.
·
Fracture
is conchoidal.
·
Hardness
is 7, less in cryptocrystalline forms.
·
Specific
Gravity is 2.65 or less if cryptocrystalline. (average)
·
Streak
is white.
--------------------------------------------------------------------------------------------------
·
Other
Characteristics: striations on prism faces run
perpendicular to C axis,
piezoelectric (see tourmaline)
and index of refraction is 1.55.
·
Associated
Minerals: Are numerous and varied but here are some of
the more classic associations of quartz (although any list of associated
minerals of quartz is only a partial list):
amazonite a variety of microcline,
tourmalines especially elbaite, wolframite,
pyrite,
rutile, zeolites, fluorite,
calcite,
gold, muscovite,
topaz, beryl, hematite and
spodumene.
·
Best
Field Indicators are first the fact that it is very
common (always assume transparent clear crystals may be quartz), crystal habit,
hardness, striations, lack of cleavage and good conchoidal fracture.
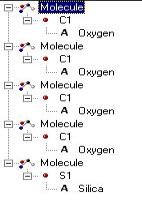
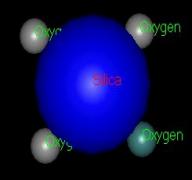 Pauling
Rule: Using the theories
proposed in the section on pegmatite of this study, with adulteress material
being absent, brings us to a level of purity in the quartz. Pauling rule as
discussed in the book, Physical Geology, implies the perfect balance of ions
within silica. Pauling rule also discusses the precise formulation of
two-consideration eletroneutrality and geometric packing. Stable crystal
structure result from the interplay of these two considerations. Optical quartz
is considered stable because of its eletroneutrality and Model
By: Bradstreet
Pauling
Rule: Using the theories
proposed in the section on pegmatite of this study, with adulteress material
being absent, brings us to a level of purity in the quartz. Pauling rule as
discussed in the book, Physical Geology, implies the perfect balance of ions
within silica. Pauling rule also discusses the precise formulation of
two-consideration eletroneutrality and geometric packing. Stable crystal
structure result from the interplay of these two considerations. Optical quartz
is considered stable because of its eletroneutrality and Model
By: Bradstreet
geometric packing. The arrangement of atoms is noted as four
oxygen ions around one silica ion. source
for Paulings rule (Deffeyes, Hargraves, Judson, Physical Geology 1976. p.44).
|
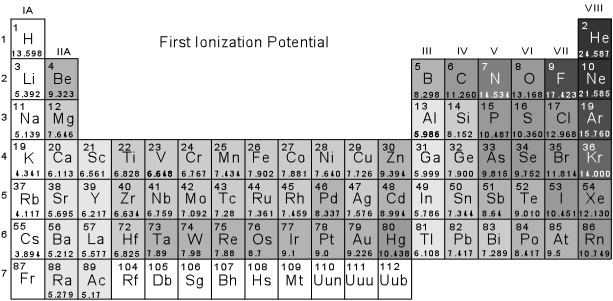
Ionization Potential When electrons are either removed or gained by an atom there is a transfer of energy. The amount of energy required to remove an electron is called the ionization potential. The version of the periodic table shown below gives value of the first ionization potential. Note that elements with high ionization potential do not like to give up electrons, while those with low ionization potential can give up electrons more readily and tend to become cations. We can make the following observations: |
|
|
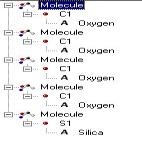
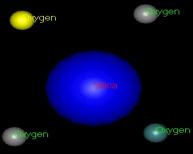
Group IV
elements tend to lose 4 electrons to
become +4 ions (i.e. C+4, Si+4,
Ge+4). But Pb,
usually only
loses 2 electrons to become Pb+2.
Model shows the silica losing its oxygen then becomes +4 ion ( Model by: Bradstreet)
|
·
The Noble gases all have
very high first ionization potentials, indicating that their electronic
structure is stable. A glance at the periodic table showing filling shells
(above) indicates that the Noble Gases all have in common completely filled p
- orbitals. It is because these sub-orbital shells are full that these
elements do not readily become ions and do not easily combine with other
elements to become compounds. |
Crystal Chemistry provided By: Prof. Stephen A. Nelson, Tulane University,
Geology 211, Mineralogy,
http://www.tulane.edu/~sanelson/geol211/index.html#Links, last modified on
10/7/99
How did these conditions reach the optimum environment for
optical quartz?
Upon examination of optical quartz in the LaChance quarry,
using the ionization potential within
the bounds of crystal chemistry, we can assume the equilibrium of the quartz
core was at its optimum level, upon solidification. Using the ionization table above, one can observe
that Silica (Si) has a high resistance in giving up its electrons, which only
further contributed to the equilibrium of the core in the Lachance quarry. In contrast the
Thopsom, and Alburn area pegmatite’s revealed Fractionation and replacement
minerals. The Fractionation produced a very unstable environment and the forces
where great enough that the atoms could not arrange together to form any kind
of core. The observations made in this area are that these rocks underwent
great pressure; hence the evidence of later intrusions is clear. It really
comes down to the fact that adulteress material is the chief component in making
these surrounding pegmatites impure. In contrast to this negativity, these
impurities (later magma injections) were the building blocks for rare earth
elements.
More
discussion on solidification: Remembering the process of solidification took place over a
temperature range that spanned hundreds or perhaps thousands of years. It is
hard to conceive that this rock formation remained stable, during all the
volcanism of this period. However if the proposed theory is correct what did
remain stable is the constant balance [uninterrupted by later injections] of
solutions. Fractionalization occurred within this area due to tremors,
earthquakes, and the shifting of near by rock bodies that had later injection.
The pressure of these later injections then squeezed the already hardened rock
creating fractionalization within the rock structure. With this knowledge it is
easy to understand the reason why large bodies of uninterrupted quartzes are
sparse.
Past studies: The studies presented
by the Schenectady Museum Archives, revealed that Elihu Thomsom achievements
while building the refractive mirrors was a stepping stone in quartz history.
His work brought forth many new question and research into the area of silica.
The work was well noted for the annealing process of fused silica. He also
discovered Pyrex during the process.
“During and after World War II, the Army Signal
Corp sponsored and subsidized a comprehensive program to develop domestically
grown quartz. The program was successful and resulted in the commercial
production of cultured grown quartz in 1958” (United States Bureau of Mines,
1975. p. 881). This was the development of the autoclave and “by 1975 there
were 475 operating in the United States” (United States Bureau of Mines, 1975.
p. 881). The autoclave gave rise to the production of reliable quartz that
could be processed much quicker and have a high-grade value.
In today’s technology silica has become an
intricate part of our society. Changing the way the world communicates, as with
remarkable discovery in medicine. Communication has now expanded through the
use of the computer (silicon chips), this vast network of chips per-say has
ensured the stability of communication.
The aid of the computer has touched almost every
industry on the planet. Uses include communication, information management,
molecular, electrical, aerodynamics, and quantum physics simulation…etc. Using
simulation as an aid to science has enabled predictability within research,
further ensuring the safety of the design phase; saving lives and money.
One remarkable discovery worth noting is the
restoration of eye site by silicon chips.
In landmark surgeries
at the University of Illinois at Chicago Medical Center on June 28, the first
artificial retinas made from silicon chips were implanted in the eyes of two
blind patients who have lost almost all their vision because of retinal
disease. Both patients were released from the hospital the following morning.
Preliminary tests determined that no complications had occurred. A third patient
received an implant June 29 at Central DuPage Hospital, in Winfield, Illinois.
(ScienceDaily Magazine, 2000).
The list of uses for
silicon within our society is vast and has made remarkable changes within the
evolution of man. With the knowledge behind us in regards to silicon one can
only imagine the future! If it had not been for the first pioneers
investigating into the rock around us we may not have had the ability and
luxury of today’s technologies.
Summary
Tying
all the data together: Many that study this area of pegmatites have different
theories and conception in respect to the Sebago Batholith and its surrounding
pegmatite. The field of geology is highly speculative, but there are
indisputable mechanism and forces that science has unraveled.
The
event, the Sebago Batholith, set in motion the mechanism of creation for its surrounding
pegmatite. It is conceivable that separate plutons where injected into the
country rock that surrounds the Sebao Batholith. These surrounding plutons are
relatively the same in composition as the Sebago Batholith, but the pegmatites
that formed within these granites are somewhat different. The different
composition can be contributed to many variables. These variables are: distance
the magma traveled, different rock formation the magma traveled through, time
cooling, rapid cooling agents, pressure, later injections of magma, and mineral
compositional changes; that these magma underwent while traveling.
Reiterating the key issues: Many
agree that magma emanating from the core can form numerous chambers that spread
of many kilometers of country rock. Also many agree the wall rock is re melted
adding compositional changes to the solidified magma.
This study has
concerned itself with many aspects of geology. Its focus is the occurrence of
optical quartz at LaChance quarry. Quartz, SiO2, (2 parts oxygen and one part silica) that is found at the
LaChance quarry is optimally balance. The eletroneutrality and geometric
packing are necessary components when determining the optical value of quartz.
Conclusion
of the study: It is certain that optical
quartz is present in Maine’s pegmatite. What conditions made this possible is
what this study has tried to unravel. It would be presumptuous to say that one
contributing fact was the cause of theses conditions. Geoscience and
Geochemistry has enabled us to narrow the agents of creation.
These
agents are:
·
Re-injections of magma into
the smaller chambers surrounding the Sebago Batholith, which supplied the chambers
with more oxygen, upon cooling
·
Magma becoming adulterated as
it forced its way through the country rock
- Temperature
fluctuation vs. temperature stability
To obtain optical quartz the oxygen within
silica needs to have a perfect balance one-part silica and 2-parts oxygen. It
is when quartz is off balance, that quartz becomes milky having non-optical
properties. Silica that becomes adulterated either changes completely to
another mineral or it becomes colored, depending on the agent imposed on the
silica. If quartz cools at a slow rate, uninterrupted it is more likely that
the quartz will remain stable then if it cools at a rapid rate. This is because
rapid cooling is generally produced by other elements interacting with the
silica, hence adulterated the silica.
One could conclude that the quartz found at the
Lachance quarry in Brunswick Maine was un-adulterated and remained stable until
complete solidification could take place.
Bibliography and Work Cited
Below is the Information that assisted in this study
Behn,
Mark D, Eusden, Dykstra J. JR., and Notte, John III A, A Three-Dimensional
Gravity Model of the Southern
Contact of the Sebago Pluton. Maine
Department of Geology, Bates
College, Lewiston
http://www-geodyn.mit.edu/mark/CJES_1998.html
This web site
posting shows the gravitational settling of the Sebago Pluton. It also
show the orientation of the Pluton
itself
Cameron, Eugene N, 1942-45, Pegmatite Investigations 1942-1945
New
England Geological Survey.; Professional Paper 255.
United States Government Printing Office, Washington :
The report is based on work done
jointly by: Eugene N. Cameron, David M.
Larrabee, Andrew H. McNair, James J.
Page, Glenn W. Stewart, and Vincent E.
Shainin
Cox, Keith, Gordon, Bell D. J, and
Pankhust R, J, 1979, The Interpretation Of Igneous
Rocks. Ed. George
Allen & UNWIN LTD, Printed: William Clowes & Sons
Limited,
The reference
supplies a general knowledge of igneous rocks, has been used here to
substantiate collaborational view of igneous rocks.
Deffeyes, Kenneth, Hargraves Robert, and Judson, Sheldon.
Physical Geology. 1976. ED.
Pretince-Hall, Inc New York, Princeton University
This book is an introduction to Physical Geology. It has been written for the use of both majors and non-majors, in a one semester or one-term at the college level
Doyle, Robert, 2000, Professor of Geology
at University of Maine, Augusta, Previously server has Maine State Geologist,
Francis, Carl A, and Wise, Michael, A, 1992, Distrubution, Classification and Geological
Setting of Granite Pegmatities in Maine; Notheast Geology, Vol. 14 No. 2&3.
(82-93 p)
Michael Wise Department of Mineral
Science, Smithsonian institution washington DC USA 20560 and Carl Francis
Harvard Mineraology Museum Cambrige, MA 02138
Geologic, Bedrock Map Maine. 1985, Department of Conservation. edited, Philip
H. Osberg University of Orono, Arthur M. Hussey Bowdoin College, Gary M, Boone,
Syracuse University
Hussey Arthur M, II,
1981, Bedrock Geology of Lower
Androscoggin Valley – Casco Bay Area; Maine Geological Survey. Open File
#No. 81-29
This
report is a preliminary and has not been edited or reviewed for conformity with
Maine Geological Survey standards
Hussey Arthur M, II, 1983, Bedrock Geology of Lewiston 15-minute
Quadrangle, Maine; Maine Geological Survey, Open File #No. 81-29
Lachance,
Geoffrey. July 2000, Personal Interview. 16 July. 2000, owner of LaChance
Quarry
The Mineral Gallery: ,1998 by Terran Technologies, Inc. All rights reserved.
http://mineral.galleries.com:/minerals/SILICATE/QUARTZ/quartz.htm#p
Note: Unless otherwise noted, all mineral
descriptions and images, plus the related descriptions on this server is the
property of Amethyst Galleries, Inc., and may not be copied for commercial
purposes. Permission to copy descriptions and images is granted for personal
and educational use only. All such copies must include this copyright notice
and explicit references to the URL http://mineral.galleries.com/.
All
programs and data structures are Copyright © 1995,1996,1997,1998
Laverdiere,
Gary, July 2000, Personal Interview: 20 + years of pegmatite investigation
Maine
Geological Survey, 1957 Maine Pegmatite
Mines And Prospects & Associated
Minerals. Mineral Resources
Index No. 1. State Geologist: John Rand,
Department of Development of
Industry and Commerce
Präzisions
Glas & Optik. Quartzglass Components
http://www.pgo-online.com/intlframes/uebersichtset.html.
Date access, July 2000
This online
documentation gives a brief overview about standard products and
standard optical glass types.
Press,Frank
Siever, Raymond, 1997, Understanding Earth / Frank Press, Carneie
Institution of Washington Raymond Siever Harvard university -2nd ed. First
printing, 1997, by: W. H. Freeman and
Company
ScienceDaily Magazine, 2000, (Source:
University Of Illinois At Chicago
(http://www.uic.edu) Date: Posted
7/3/2000)
Http://www.sciencedaily.com/releases/2000/07/000703101426.htm
In landmark surgeries at the University of Illinois at
Chicago Medical Center
on June 28,
Thomsom,
Elihu. 1931, GE Scientist and Engineer, MIT Graduate: Served on Board of
Directors. Schenectady Museum Archives, Archivist: Chris
Hunter, GE Historical
Research Department
Tucker, Robert, D, and Marvinney, Robert, G, 1989, Studies in Maine Geology
Ingeous and
Metamorphic Geology. Vol #3. Department of Conservation
United
States, Bureau of Mines, 1975, Mineral Facts and Problems. Ed. Staff, Bureau of
Mines. Washington: The Bureau: for
sale by the Supt. Of Docs. U.S. Govt. Print
Off. {1976}
1,266 P.: 203
diagrs cm (bulletin – bureau of mines: 667 ISSN 0082-9129) 1.
Mines and mineral resources- United States 2. Mineral Industries- United
States I.
Title: (series: United States. Bureau Of Mines. Bulletin – bureau of
Mines; 667)
This reference can be found at Bates College Library, in Brunswick Maine
where
a extenxise collection of Bureau of Mines collection is housed